
Production
-
Basics (Click for more)
- Publications
- Technical Notes
- Quality Control Plan
- Small Business Resources
- Videos
-
Tools (Calculators)
-
Literature
- Links
Biodiesel Chemistry
It is not necessary to be a chemist to understand where biodiesel comes from and how it is used. However, it is useful to review some of the fundamental chemical principles that are behind biodiesel so that its properties can be understood.
Vegetable oil and Animal Fat
All vegetable oil and animal fats consist primarily of triglyceride molecules as shown schematically below.
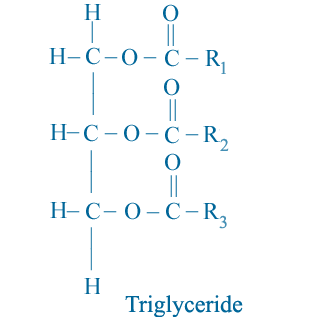
R1, R2, and R3 represent the hydrocarbon chain (such as alkyl group) of the fatty acid elements of the triglyceride. Note that there is a three-carbon chain called the glycerol backbone that runs along the left side of the molecule. Extending away from this backbone are the three long fatty acid chains.
Fatty Acids
Fatty acids are Carboxylic acid with at least four carbons in the chain. The fatty acid in their free form, have the configuration shown below:
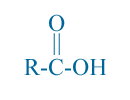
here R is a hydrocarbon chain (Could be one of the chains R1, R2, or R3 from vegetable oil) and the function group -COOH. Fatty acids are names has -ic suffix. Butyric acid is the one with four carbon atoms as
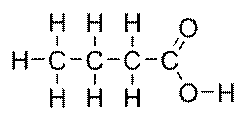
The properties of the triglyceride and the biodiesel fuel will be determined by the amounts of each fatty acid that are present in the molecules.
Instead of full name, fatty acids are usually shorthand noted by two numbers: the first number denotes the total number of carbon atoms in the fatty acid and the second is the number of double bonds. For example, 18:1 designates oleic acid which has 18 carbon atoms and one double bond.
The notation for some of the common fatty acids are as follows:
Table 1 – Names of Fatty Acids
|
14:0 |
Myristic Acid (tetradecanoic acid) |
|
16:0 |
Palmitic Acid (hexadecanoic acid) |
|
18:0 |
Stearic Acid (octadecanoic acid) |
|
18:1 |
Oleic Acid |
|
18:2 |
Linoleic Acid |
|
18:3 |
Linolenic Acid |
|
20:0 |
Arachidic Acid (eicosanoic acid) |
|
22:1 |
Erucic Acid |
Table 2 shows the fatty acid compositions of a number of common vegetable oils and animal fats.
Table 2 – Fatty Acid Composition of Various Oils and Fats.
|
Oil or fat |
14:0 |
16:0 |
18:0 |
18:1 |
18:2 |
18:3 |
20:0 |
22:1 |
|
height:.3in'>
Butter |
7-10 |
24-26 |
10-13 |
28-31 |
1-2.5 |
.2-.5 |
||
|
Corn |
1-2 |
8-12 |
2-5 |
19-49 |
34-62 |
trace |
||
|
Cottonseed |
0-2 |
20-25 |
1-2 |
23-35 |
40-50 |
trace |
||
|
Lard |
1-2 |
28-30 |
12-18 |
40-50 |
7-13 |
0-1 |
||
|
Linseed Oil |
4-7 |
2-4 |
25-40 |
35-40 |
25-60 |
|||
|
Olive |
9-10 |
2-3 |
73-84 |
10-12 |
trace |
|||
|
Peanut |
8-9 |
2-3 |
50-65 |
20-30 |
||||
|
Rapeseed – Hi Erucic |
3.0 |
0.8 |
13.1 |
14.1 |
9.7 |
7.4 |
50.7 |
|
|
Rapeseed – Hi Oleic |
4.3 |
1.3 |
59.9 |
21.1 |
13.2 |
|||
|
Safflower – Hi Linoleic |
5.9 |
1.5 |
8.8 |
83.8 |
||||
|
Safflower – Hi Oleic |
4.8 |
1.4 |
74.1 |
19.7 |
||||
|
Soybean |
6-10 |
2-5 |
20-30 |
50-60 |
5-11 |
|||
|
Tallow |
3-6 |
24-32 |
20-25 |
37-43 |
2-3 |
|||
|
Tung Oil |
3-4 |
0-1 |
4-15 |
75-90* |
||||
|
Yellow grease |
1.27 |
17.44 |
12.38 |
54.67 |
7.96 |
0.69 |
0.25 |
0.52 |
What are esters?
Esters are a type of chemical compound that contains the following grouping of
carbon hydrogen and oxygen:
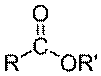
Compare this to fatty acid, the hydroxyl group(-OH) group is replaced by (-OR'), where R' is a hydrocarbon chain. The name of the ester is derived from R' and R-COOH with suffix -ate . For instance if R' is a methyl group (-CH3), and if the ester is derived from Butyric acid, then the ester is called Methyl Butyrate. Another example is methyl palmitate, which is derived from palmitic acid.
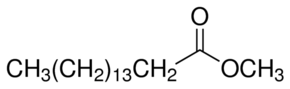
Here the CH3 designates this as a methyl ester while the rest of the molecule represents the palmitic acid. Since this molecule has only one R' group it is called mono-alkyl-ester or mono-ester. Biodiesel is nothing but mono-alkyl esters of long chain fatty acids derived from vegetable oils or animal fats.
Other examples of ester molecules are:
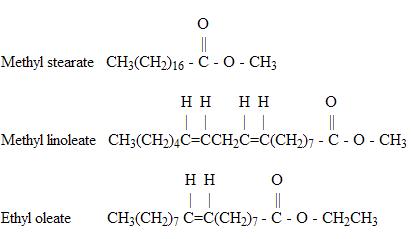
Note that each of these example contains only one occurrence of the ester functional group.
All of these examples are known as monoesters. Other organic molecules can contain more than one occurrence of the ester group, such as the triglyceride shown below:
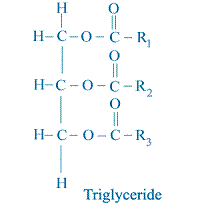
Note that this molecule contains the ester group three times. Thus, it is called a triester. Sometimes people refer to the reaction that converts oil or fat (triglyceride) to methyl esters (biodiesel) as “esterification” as if the reaction were converting something that is not an ester into an ester. In reality, the reaction converts one type of ester into another type of ester. This is why the reaction is more properly known as transesterification.
Transesterification
Transesterification is the process of reacting a
triglyceride molecule with an excess of alcohol in the presence of a catalyst
(KOH, NaOH, NaOCH3, etc.) to produce glycerin and fatty esters. The chemical
reaction with methanol is shown schematically below: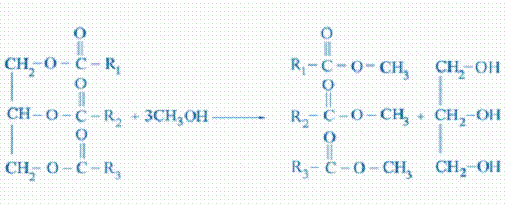
Triglyceride + methanol mixture of fatty esters + glycerol
In this transesterification reaction, one mole of triglyceride reacts with 3 moles of methanol to produce three moles of fatty esters (biodiesel) and one mole of glycerol. On a weight basis, the mole of triglyceride will weigh about 884 grams, the three moles of methanol about 96 grams, the resulting three moles of biodiesel will weight about 888 grams and the mole of glycerol will weigh 92 grams. Most biodiesel producers will add from 1.6 to 2.0 times the chemically correct amount of methanol to ensure that the reaction is driven to completion. This means that a substantial amount of methanol will be left in the products that should be removed and recovered. Expressed on the basis of 100 grams of oil, the mass balance for the case of 100% excess methanol becomes:
100 g soybean oil + 21.7 g methanol 100.4 g biodiesel + 10.4 g glycerol + 10.9 g methanol
Reference
Peterson, C.L., “Vegetable Oil as a Diesel Fuel: Status and Research Priorities,” ASAE Transactions, V. 29, No. 5, Sep.-Oct. 1986, pp. 1413-1422.
Linstromberg, W.W., Organic Chemistry, Second Edition, D.C. Heath and Company, Lexington, Mass., 1970.
Tat, M.E., and J. H. Van Gerpen, “Fuel Property Effects on Biodiesel,” ASAE Paper No. 036034, American Society of Agricultural Engineering Annual Meeting, Las Vegas, NV. July 27-30, 2003.
The dominant fatty acid in tung oil is a conjugated isomer of linolenic acid called eleostearic acid. The three double bonds in eleostearic acid are located at 9:10, 11:12, and 13:14 instead of at 9:10, 12:13 and 15:16 as in linolenic acid.
Tweet